
Daniela Kaufer
Integrative BiologyDaniela Kaufer is an Associate Professor in the Department of Integrative Biology and the Helen Wills Neuroscience Institute at UC Berkeley. She was born and raised in Israel where she studied Biological Sciences at Technion, and received her Ph.D. in Molecular Biochemistry at Hebrew University. She was a Human Frontiers Post-doctoral Fellow at Stanford University, before joining the UC Berkeley faculty in 2005. Daniela’s lab studies brain plasticity throughout life in face of stress and neurological insults, with a particular focus on plasticity involving adult neural stem cells and across the neurovascular unit – neurons, astrocytes, oligodendrocytes and the components of blood brain barrier.
Spark Award Project
Blood-Brain Barrier Dysfunction as a Biomarker and a Therapeutic Target for the Prevention of Acquired Epilepsy
The protection of the brain from blood-borne toxins, proteins and cells is critical to the brain’s normal function. Insults to the brain, including traumatic brain injuries, stroke, infections, and brain tumors are associated with Blood-Brain Barrier (BBB) dysfunction, and significant increase in the risk for epilepsy. However, there is no clinically applicable strategy to identify at-risk patients, or prevent the development of epilepsy in them. Daniela Kaufer, in collaboration with Dr. Alon Friedman from Ben Gurion University, will build on previous findings demonstrating that the blood protein albumin activates TGFß signaling cascade in astrocytes and initiates an epileptogenic process following BBB compromise. Kaufer and Friedman have demonstrated that blocking TGFß signaling following injury prevents the development of epilepsy, and identified an FDA-approved drug that effectively blocks the development of epilepsy and eliminates delayed seizures following BBB insult in rats and mice. Now they propose a preventive approach that includes screening BBB integrity as a marker to identify at-risk patients, and administration of losartan or alternative TGFß signaling blockers to prevent epilepsy development in these patients.
Daniela Kaufer’s Story
A few years after serving in the Israeli army during the first Gulf War, Daniela Kaufer made a startling discovery about the effect of psychological stress on the brain. As a graduate student at the Hebrew University she showed that the kind of extreme stress experienced in combat can break down the physiological barriers that normally protect the brain.
She could not have known it then, but the finding would eventually lead her to uncover a key change in brain chemistry that triggers epileptic seizures. The Bakar Fellows Program is now helping her refine a strategy to block the threat and protect the brain from damage caused by physical trauma and other insults.

A physiological line of defense normally prevents circulating blood from entering the brain. Known as the blood-brain barrier, the tightly controlled system buffers the brain from exposure to bacteria and other blood-borne invaders. Kaufer’s research has revealed how brain trauma can disrupt brain function once the barrier is breached.
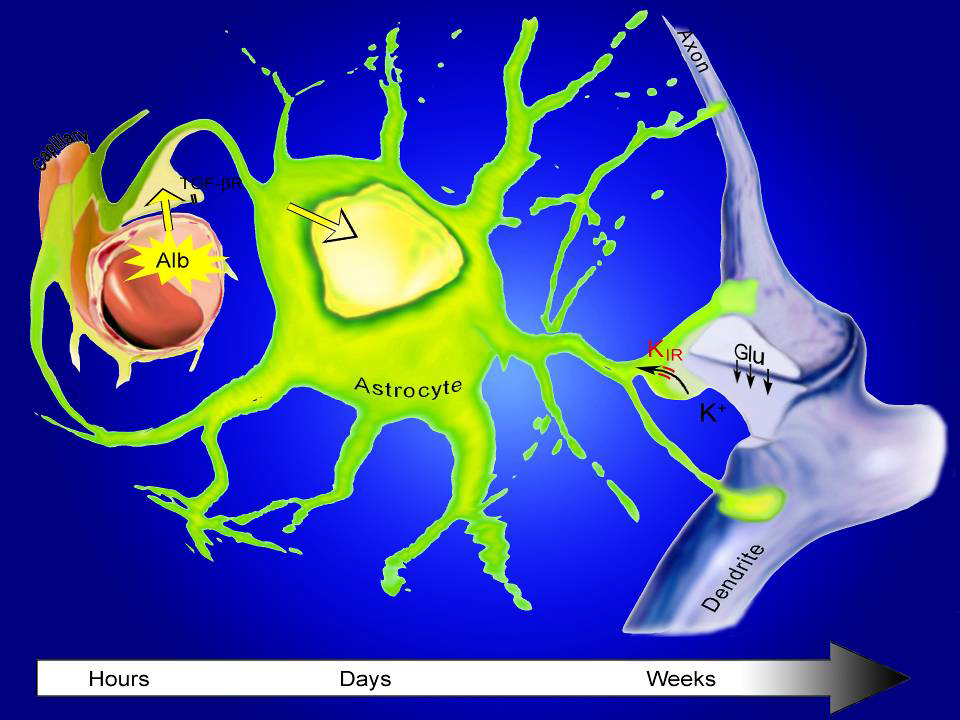
In lab research as a postdoc at Stanford in 2002, Kaufer and her Israeli colleague Alon Friedman examined what happens in the brain when the barrier is compromised. They found that seizures were likely if – and only if – the brain came in contact with blood that had been circulating in the body.
They showed that a very common protein in blood called albumin accelerates signaling between neurons to abnormal levels. Neurons become overexcited and can cause seizures.
“We were surprised, even a little disappointed, that it was such a common component of the blood – nothing exotic at all – that led to epilepsy,” recalls Kaufer, associate professor of integrative biology.
She and Friedman went to on to show that albumin interacts with a ubiquitous cell protein called TGF-Beta receptor to cause the damage.
In the healthy brain, TGF-Beta signaling affects activity of star-shaped sister cells of neurons called astrocytes, which normally limit neuron-to-neuron firing signals across the synapse. But when albumin stimulates TGF-Beta receptors, astrocytes lose some of their control. Neuron signaling spikes dangerously, and promotes the development of epileptic seizures.
“Researchers knew that following traumatic brain injury the risk of epilepsy was great, but they didn’t know why,” Kaufer says.
As luck would have it, a prescription drug for hypertension blocks TGF-Beta signaling. With support from the Bakar Fellows program, Kaufer is now carrying out research to confirm that blocking abnormal TGF-Beta activity can prevent epilepsy from a range of insults.
She expects that her and Friedman’s lab research, coupled with clinical studies, will demonstrate the drug’s ability to protect the brain and move it into use in emergency medicine to prevent victims of brain trauma from becoming epileptic.
Kaufer and Friedman’s research is suggesting too that a number of assaults besides physical trauma – from brain infections to stroke – can also weaken the blood-brain barrier, and lead to the development of epilepsy through TGF-beta signaling. Emergency medicine physicians need only determine if the barrier has been breached to know if a patient is at risk for seizures.

Fortunately, the condition of the blood-brain barrier can be assessed using a safe and straightforward FDA-approved MRI protocol, so screening for epilepsy risk is within reach, says Kaufer.
“Right now, if someone comes to the emergency room with traumatic brain injury, they have a 10 to 50 percent chance of developing epilepsy. But you don’t know which ones, nor do you have a way of preventing it. And epilepsy from brain injuries is the type most unresponsive to drugs.
“I’m very hopeful and that our research can spare these patients the added trauma of epilepsy.”