
Roya Maboudian
Chemical and Biomolecular EngineeringRoya Maboudian is a Professor in the Department of Chemical and Biomolecular Engineering.
Project Description
Carbon Dioxide Sensing for Indoor Air Quality Monitoring
Inexpensive and efficient carbon dioxide detectors are desirable for many applications, including air quality assessment, food storage, microbial investigation and patient care. The infrared sensors most commonly in current use are expensive and remain difficult to miniaturize. With her Bakar Fellows Spark Award, Roya Maboudian will produce an inexpensive alternative based on colorimetric sensing. These sensors will be simple and user-friendly, exhibiting a color change visible to the human eye, yet sensitive enough to detect minor variations useful in air quality monitoring. They will be fabricated from a porous crystalline class of materials, called metal organic frameworks, composed of metal clusters connected by organic linking molecules. This material system can be tailored to strongly interact with carbon dioxide, and by incorporating a dye that changes color based on the amount of bound gas, a highly sensitive CO2 detector can be realized.
Roya Maboudian’s Story
While rising carbon dioxide levels in the atmosphere cause great concern worldwide, most of us pay little attention to risks posed by CO2 changes indoors.
CO2 concentration in fresh air is about 400 parts per million (ppm). But get a group of people packed in a closed indoor space, and CO2 concentration can rise quickly. Recent studies suggest that as levels increase above 1,000 ppm, decision-making and other cognitive abilities decline.

Roya Maboudian, Professor of Chemical and Biomolecular Engineering, studies the properties of nano-materials, including how their surfaces affect their performance. As a 2019-2020 Bakar Fellow, she is developing small, inexpensive and sensitive CO2 sensors. She described her research and its potential.
Q. Before people start having cognitive problems from high indoor CO2 levels, how else are they affected?
A. When concentrations in a room are too high, people become drowsy and their attention starts to lag. High CO2 levels are suspected to play a role in “sick building syndrome,” which includes symptoms like shortness of breath, dizziness, headaches and more.
Q. What causes the CO2 levels to increase in-doors?
A. We naturally produce CO2 as we breathe. Exhaled breath contains about 40,000 ppm of CO2 (1000 times higher than in outside air). So, without adequate ventilation, the CO2 concentration increases indoors.
Q. How are CO2 levels detected now?
A. The most commonly used device is called a nondispersive infrared sensor, or NDIR. They’re accurate, but they are difficult to miniaturize, they require electrical power to operate, and they’re expensive – hundreds to thousands of dollars.
Q. How do the sensors you are developing address that problem?
A. They are small and don’t require electrical power. Each person could wear an individual sensor in the form of a small badge. They would be very useful in areas where many people congregate or work together, like in an office or a classroom.
Q. How do the new sensors work?
A. They’re easy to operate — in fact there is nothing to operate. They indicate changing CO2 levels by changing color, like pH strips we are familiar with. If the color indicates a high level of CO2 the person would know that they need to open a window, go outside or adjust the building HVAC.
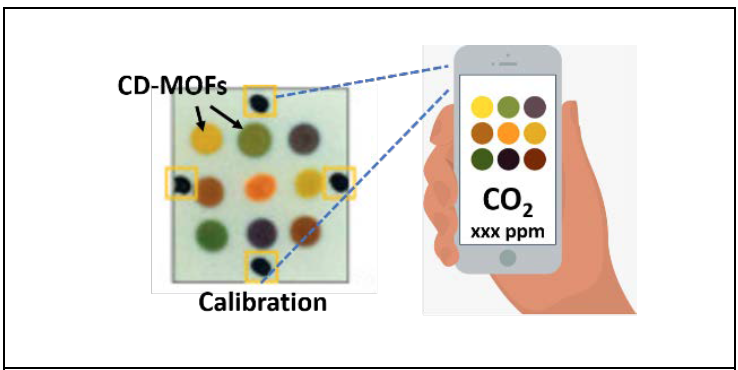
Q. What accounts for their small size and sensitivity?
A. Sensitivity is directly related to the surface area and the chemical affinity of the material for CO2. Our sensor is based on a porous material that has high surface area and strong affinity to CO2. As the CO2 interacts with this material, it forms an intermediate molecule. This molecule reacts with a third molecule, called a pH indicator, which changes color depending on the CO2 concentration. The sensor operates without any electrical power. High surface area also means we need very little material, so the sensor can be very small and lightweight.
Q. How sensitive are they compared to the expensive NDIR sensors?
A. They are currently not as sensitive or quantifiable as the NDIR technology. We need to more precisely link color change to CO2 levels. Our objective is not to compete with NDIR sensitivity, but to provide a more qualitative measure of CO2 for situations where size, power and price are critical, as in monitoring for individuals.
Q. They are simpler and smaller than the NDIS sensors. It seems they would not cost as much to produce and use.
A. That’s right. They are made of inexpensive nanomaterials that can be placed on a cheap substrate such as a filter paper. So, we expect that they would be very economical.
Q. What do you think is the market for small, individual CO2 sensors?
A. There is a growing interest in people wanting to know what they are exposed to, both at work and at home. Parents are concerned about their children’s exposure. Employers wants to insure a healthy and productive workplace for their employees. It’s impractical to carry a large number of NDIR sensors, each the size of a small book.
Q. What work needs to be done before they are ready for commercialization?
A. Of course, the sensors are not as simple as they sound. Adrian Davey, our Bakar Innovation Fellow, is measuring the sensitivity to CO2 concentrations typical of indoor air and quantifying the color response.
We are also concerned with the stability of our porous material. How long will the material function? What is its shelf life?
These are critical to demonstrate the sensors’ commercial potential.