
Russell Vance
Molecular and Cell BiologyRussell Vance is a Professor of Immunology and Pathogenesis in the Department of Molecular and Cell Biology.
Project Description
Can We Harness Virus-Triggered Immunity for Cancer Immunotherapy?
Immunotherapy has revolutionized the treatment of cancer. However, many patients fail to respond to current cancer immunotherapies. Recent data suggest that immunotherapy resistant tumors lack infiltration of the immune cells necessary for therapeutic responses. One of the characteristics of these so-called “cold” tumors is the absence of a gene expression signature characteristic of type I interferons, a class of secreted signaling proteins that elicit potent anti-tumor immune responses. Russell Vance’s lab will identify the pathways that silence type I interferon production in tumors and develop novel small molecule inhibitors of these pathways, to unleash anti-tumor interferon responses. This approach is unique as its goal is to reverse the silencing itself, rather than to induce interferon expression within a tumor.
Russell Vance’s Story
A breakthrough in cancer treatment just nine years ago has brought new hope to millions of people whose cancers are resistant to all other therapies. The new treatment, cancer immunotherapy, frees the body’s potent immune system to attack tumors.
Cancers are our body’s own cells, mutated to fuel aggressive growth and camouflaged with surface proteins to deceive the immune system and quell any defensive response. The breakthrough treatment releases the brake on the immune system, unleashing its army on cancerous cells.
But not all cancers respond to immunotherapy, and researchers around the world are struggling to find out why.

Russell Vance, PhD, Professor of Immunology and Pathogenesis in the Department of Molecular and Cell Biology, studies the immune system’s production of Interferons, a type of protein that normally helps trigger the immune response to viruses.
He suspects that cancer shields itself from immune attack at least in part by reducing the amount of interferon processed by the immune system. With support from the Bakar Fellowship Program, he is developing a way to disable cancer’s ability to block interferon production.
Dr. Vance describes his novel approach.
Q. Why do you think cancer immunotherapy has been able to treat many types of cancer but not all?
A That’s the million dollar question. Cancer immunotherapy successfully treats melanoma, lung cancer, and several other kinds of cancer. But even with these types of cancers, it doesn’t work for all patients. Often, less than half respond to the treatment. The reasons why the therapy doesn’t work in all cases is not well understood. There probably are several reasons – maybe even in the same patient.
Q. Why do you think boosting interferon production will knock out cancer?
A There is some good evidence in mouse models that stimulating interferon clears a tumor, although this has not been proven in humans yet.
Q. What is the thinking behind your strategy?
A Many labs are trying to figure out ways to induce an interferon response. They want to fight the tumor by ramping up the immune system’s production of interferon. We propose the opposite. Our idea is that tumors evolve to silence the production of interferon. We propose to reverse what tumors do. We want to turn off the tumor’s ability to block interferon production. We want to silence the silencer.
Q. What progress have you made so far?
A couple of years ago, we decided to use CRISPR gene editing to mutate thousands of genes in human cells, screening them to identify ones that regulate type 1 interferon production. We identified a protein that inhibits the normal interferon response. Deletion of the gene encoding this protein results in a 10,000-fold increase in interferon 1 production.
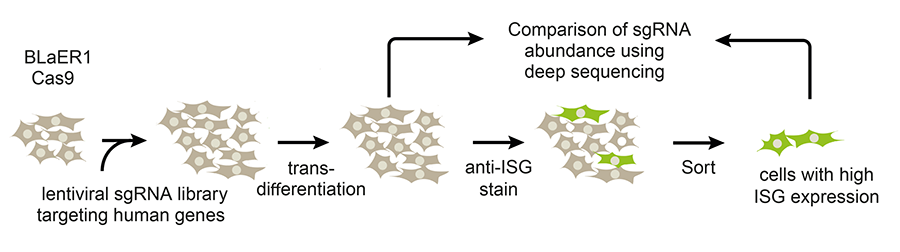
Q. How can you translate the discovery to improve cancer immunotherapy ?
The Bakar Fellowship will support our research on two parallel tracks. We will carry out laboratory studies to confirm that the interferon response we observe will lead to the destruction of tumors. At the same time, we’ll screen thousands of molecules to identify a potential drug that will specifically silence this new protein in humans and thereby unleash the anti-tumor interferon response.
Q. How do you screen a hundred thousand proteins to find that potential drug – a needle in the haystack proposition?
A This is now a standard technology that drug companies use all the time. We will find expert collaborators to help optimize the necessary assays.
Q As your research progresses, how would it make the transition into clinical treatment?
A Our work is still in the very early stage. It wouldn’t get NIH support because it would be considered high risk. What’s great about Bakar Fellowship is that they are willing to take that chance.
The Bakar Fellows Spark Award allows us to conduct the proof-of-principle experiments that we’ll need to interest additional funders or investors. And by joining the Bakar Fellows community, we have the opportunity to interact with other entrepreneurs and investors.
There is a huge amount of interest in immunotherapy. If we generate good preliminary data with our Bakar Fellows Program support, we would be in good position to partner with companies to translate our findings into effective therapies.